9
2011-04-08
Göte Bertilsson
A
continuing project. Please comment and contribute.
Soil and crop management for
today and tomorrow.
This is not
a basic textbook. It is an attempt to summarize and integrate knowledge
and ideas for our present situation, where
the challenge is to meet both production demands and environmental goals at a
global level.
Introduction.
We have
deep knowledge in soil and crop science
as well as in environmental sciences and ecology. It forms a base for advanced models on the behaviour of soils and
crops, the growth processes and environmental consequences of various
kinds. Continuous development is going
on, The main problem is the variability of
soil and weather parameters.
We have field experiments giving a base for recommendations
to farmers.
This is
transformed to actions in the fields, by advisors and farm managers. Their main task and interest is to optimize the
economic result of the farming enterprise.
We also
have a global and political environment listing demands or in any case
environmental problems to work on:
Food
production should increase
Water is
scarce
Climate
gases should be reduced
Nitrogen
flows should be reduced.
Chemicals
in general should be reduced
Agricultrual
land may be needed for bioenergy
Biodiversity
needs consideration.
The
practical agricultrure is influenced by economic factors, various
administrative measures and, not least, by the knowledge and interest of the
farmer
The
intention with this work is to try to summarize and discuss this complex and
ways to handle it.
The base: the soil.
In fact:
soil is not needed for growing crops. It can be done in water culture, in
mineral wool or gravel beds. But in that case continous control of water and
nutrients is needed.
With the
soil as base, the plant almost always has water and nutrients available, more
or less. The soil is a wonderful and complex system, almost a living organism.
A skeleton
of mineral particles gives support and creates a porous system which can store
water and allow passage of air and other gases.
Organic
matter, humus, combines with the soil particles and builds up a structure favouring water characteristics
and giving an environment friendly for root growth.
Living
organisms, from bacteria and fungi to various animals live on plant residues
and keep the process going. They are breaking down soil organic matter and
forming new. The soil organic matter is dynamic and changes according to
environmental conditions and agricultural management.
The
minerals in the soil are subject to weathering and may release soluble minerals
giving a base for plant nutrition.
Further –
the pH of the soil and the drainage characteristics ave very important for the
function of the soil.
The soil profile.
The roots
of many crops go surprisingly deep. The roots of winter wheat, for instance,
normally are at more than one meter deepth already in the Autumn, if the
structure of the soil profile permits. This is important. A fertile,
highproducing soil has a deep active profile. That gives a more reliable supply
of both water and nutrients.
Soil fertility – of crucial importance also
today.
It might be
believed that with our technical resources with machinery and fertilizers we
should be more independent of the soil. That is only partially true. For high
yields we need a well functoning soil giving reliable supply of water and
nutrients. We can express it this way: a medium yield of 5-6 tons can be achieved even with some
temporary shortages of water or nutrients, but for 9-10 tons the function must
be on top all the time. The soil determines the possible outcome.
Threats to the soil.
Compaction.
Heavy
machinery is a threat. Soil compaction means reduced permeability for water and
air and less favourable growth conditions. Compact soil restricts root
development. In the top soil compaction can be reapaired by plowing and
tillage, but compaction of the subsoil persists for a long time. Trials with
deep cultivation have not been very encouraging. Subsoil compaction threatens
the functioning of the entire soil profile. Heavy loads on moist soils are
damaging.
Measures:
Avoid heavy
machines. For instance – there are techniques for distributing and spreading
slurry by pumping.
Avoid
traffic on moist soils.
Minimize
field traffic.
Use fixed
tracks on the fields.
Use
deep-rooted crops in the rotation.
Organic matter.
The content
of organic matter is in long-term equilibrium with the cultivation system. But
the processes are slow. A hundred years or so are needed to approach
equilibrium, at least in north European conditions.
For
Scandinavian conditions a common system with cereals and oilseeds seems to tend
to an equilibrium content of abour 1,5% organic carbon, provided straw and
residues are returned.
Leys in the
rotation give a higher value, row crops as potatoes, beets and maize a lower.
1.5%
organic carbon is too low, according to available knowledge. On soil with less
than 2% organic carbon measures to improve the organic matter content will give
yield increases.
Measures:
Leys in the rotation
Manure and
other sources of organic matter are favourable.
Cover crops
are positive, especially if they are left until late autumn or preferably
spring.
Delayed or
reduced autumn tillage in general.
High yields
are positive, especially when residues are left in the field.
Combinations
may amplify the effect, for instance high yields+residues+reduced tillage.
Lime and pH.
In Norhern
Europe we have a precipitation surplus and in general a natural leaching and
loss of lime. Crop production and fertizers are also contributing. Mostly
”acidifying” nitrogen fertilizers are used. However, the real mechanism is
complex. The acidifying effect is to a great extent caused by the increased
production and resulting increased uptake and removal of minerals from the
soil. Also biological nitrogen fixation is causing acidification.
What soil
pH is needed? That depends on the soil and the crops. Crops have different
demands and also soils react differently. For clay soils a fairly high pH
(6,5-7.5) is important for the structure and general functioning. It is
important to keep in mind that lime losses occur and need compensation. A high
pH means higher losses and a higher maintenance demand. In natural soils the pH
are often low, the lime losses low and may be in equilibrium with the
weathering of soil minerals. But every use of the system, removal of material,
leads to mineal losses and increased acidification.
Nutrients
in soil.
This is an
extensive and complicated topic. Here is only a brief overview.
Nutrients –
that is nitrogen, phophorus and potassium, but also magnesium, sulphur and
several micronutrients.
With
fertile soils we mostly mean soils rich in lime and minerals. The parent rocks
often are lime stone or shales of different kinds.
We can add
nutrients, use fertilizers. Most soils
store phosphorus very well, to some extent also potassium and magnesium. But
for nitrogen the case is different. The nitrogen in soils (most of it) is in
the organic matter, and both build up and release are governed by rate limited
biological processes. The rates do not suffice for neither efficiently taking
care of excess available nitrogen nor delivering to a demanding crop. Nitrogen
availability must be considered annually (or on shorter basis) for each crop.
Different
micronutrients need consideration. Copper and to some extent boron can be
stored in the soil. Manganese and iron are seldom quantitatively deficient, but
may be tied up as insoluble xompounds which are unavalable for the crop. Annual
consideration is needed.
The crops
also need zinc and molybdenum. For Swedish conditions the soils normally give
adequate supply, but not always. Humans and animals also need selenium and
cobalt. Our soils (Scandinavian) are ofter poor in selenium which needs
consideration. In Finland fertiizers are enriched with a low content of
selenium for human health reasons.
Also
calcium is a nutrient , but that is mostly overshadowed by its importance for
the lime situation and pH of soils. Chloride: the plant needs small amounts
which ”Nature” provides. But chloride is often important for soils and
fertilization. Most potassium fertilizers contain almost equal amounts of
potassium and chloride. In coastal areas high amounts of chloride are deposited
by rain.
Sodium –
beets give yield increases for sodium supply. The beet plant seems to remember
its origin as a shore plant on soils with high amounts of sodium and chloride.
There are also cases where chloride seems to give yield increases to other
crops.
About systems and recycling.
If residues
and refuse are recycled to the soil at least some part of the minerals taken
away will be compensated.
In Nature.
The soil
receives minerals and other substances from rain and air. Nitrogen is ”fixed”
from the air by different organisms. There is a loss by leaching and erosion.
Nitrogen and sulphur can be lost to the atmosphere. Between these proesses the
soil evolves.
With our society.
We remove
products from the soil. We have created a large ”unnatural” outflow of nitrogen
and minerals. This had big consequences in old times, although the
impoverishment of farming soils to some extent was counteracted by inclusion of
meadows in the systems. Still the societies were small and local recycling was
in any case to some extent possible. Today more than half of the global population
lives in cities without contact with agriculture and rural society. A recycling
opportunity has developed into a waste problem.
Mineral
fertilizers and lime materials compensate the soi.
There are
many reasons for improving recycling, but the difficulties are great, not only
concerning economy and logistics. There are substances and elements in the
refuse we don't want to recycle, metals as cadmium, some organic compounds as
well as infectious agents.
However, it
seems important that the recycling idea is kept in mind, and that innovative
solutions are encouraged.
The soil – aspects on environment and
sustainability.
General.
A good
function of the soil is important for both production and environent. High
yields are positive for the soil. Farming
measures aiming at long-term productivity are often win-win situations,
positive both for production and environment.
Shortterm – longterm.
Soil
compaction situations.
A hectic
day en Spring. Slurry needs to be applied before tillage and sowing.” A little
too wet but it cannot be helped. We need to sow tomorrow before the rain is
coming.”
Another
example: The tyres are equipped with
advanced technique for adjusting the pressure between road and field. But it
takes some time. ”There is no time for that today,”
Cover
crops.
Cover crops
building organic substans in the autumn are positive för the soil organic
matter and for
soil
fertility. But initally they may involve some costs and trouble. That is
directly evident, the longterm advantage has less weight, unless it is
specifically stressed.
There is a
need for better communication of the economy of the longterm aspects. Soft
arguments like ”organic matter is important” need to be translated into hard
economy to be properly considered by the farm management.
Liming.
Proper lime
status is also a win-win situation. In
most cases maintenance is necessary to counteract the lime losses by leaching
and crop mineral uptake. Liming means loss of carbon dioxide, but that is an
unavoidable consequence.
We assume a
maintenance requirement of 150 kg calcium oxide per hectare and year. Calcium
carbonate contains 56 percent calcium oxide and 44 percent carbon dioxide.
Application of 150 kg calcium oxide means that 120 kg carbon dioxide is
released and contributes to global warming. Maybe other lime materials should
be used? It does not help, it only means that the carbon dioxide is released at
the lime factory instead.
There is no
solution, unless carbon dioxide capture is installed at the lime factory. We
have to maintain the soil status, but we should not keep a higher soil pH than
necessary.
Maybe
research should investigate possibilities to satisfy the needs of especially
lime-demanding crops (sugar beets etc) without raising the pH of the entire
soil volume.
More
recycling of organic material or minerals would reduce the demand for
maintenance liming.
Plant nutrients.
The balance principle: put ecology in the first
place and the economy will follow.
The use of
fertilizers mostly is governed by economic terms: what is the cost of the
fertilizer, what is the value of the yield increase and what is the most
economical dose?
However,
the plant nutrients applied will interact with the soil-plant system and
ultimately with our whole environent.
Could we
start in another way: what do we expect the soil to deliver to our harvested
product? Could that be a first estimate of the need for nutrient application?
An example:
Winter
wheat on a good field which has in average yielded 8 tons the last two
rotations. That yield contains 160 kg nitrogen and 25 kg phosphorus (let us
stop there in this example). What reason is there for not applying 160
nitrogen? Well, last year there was a
clover seed crop, which we estimate gives an extra 50 kg nitrogen. Then the
application should be adjusted to 110. For phosphorus we have a high soil
analysis and the soil content is unnecessary high. Therefore, we apply no
phosphorus to the wheat crop. The soil delivers
The same
thinking should be continued for other nutrients.
Let us call
it the Balance Principle. Is it too simple? Too unreliable? At least it is a
subject to discuss.
A few
conditions:
It is not a
pure replacement. If considering a perhaps low yield last year and replacing
that offtake it will not work. Consider instead the actual realistic yield goal
and plan after that. Another important quesion: is the yield potential
attained? If we do not look forward there will be no progress. 8 tons is high,
but 9 is higher. Your own experience, neighbouring results and perhaps field experiments
might give informatin.
Further: is
the soil in reasonable equilibrium?
Perhaps phosphorus is low and the soil needs improvement? Then this must
be considered.
Replacement
fertilization has great limitations. It cannot improve the situation. But when
an improvement phase has matured, the situation is different. Probably a
balance principle will gain in importance. It has a solid longterm base. But it
cannot be dogmatic. It has to be adjusted according to knowledge and analyses
available and adapted to local knowledge.
Probably we
will see that the principle of economic marginal profit as a base for
fertilizer recommendations will be difficult or impossible to use in a
situation with strongly fluctuating and high prices.
However, we
cannot neglect the prices. Some principles are discussed below for respective
nutrients.
Tools for
the Balance Principle:
Fieldwise
records, preferable 10 years or so. Crops, yields, nutrient inputs. Protein
analyses give important information about the nitrogen situation and
functioning
Soil
analyses. Phosphorus, potassium, magnesium, boron, copper, pH, organic matter.
These are not needed every year, but every 5-10th. Also, control
analyses of plants or soils during the growing period to check the direct
nutritional status for for instance manganese and other nutrients.
Zero nitrogen
plots. A few square
meters without any nitrogen application shows the capacity of the soil profile
to deliver nitrogen, the system background. The uptake can be measured to get a
quantitative value or the stand can be probed by a portable sensor. Still –
only visual observations tell great deal.
Sensor guided application and other
developments in the area of precision farming.
With all
these modifications, what remains of the Balance Principle? Most important is
that that the base for the nitrogen recommendations is an estimate of the
demands of the crop, not the marginal economics. This more ecological base
gives automatically a more longterm consideration.
Nitrogen.
Nitrogen in the soil.
Organic
matter and mineralization.
The soil
organic matter is a large nitrogen reservoir. A normal agricultural soil in
Northern Europe contains 5 tons of nitrogen per hectare in the soil organic
matter, give or take a few tons. The organic matter is broken down by soil
organisms, the nitrogen is mineralized, which releases 50-100 kg nitrogen per
year for the crops to use or as loss to the environment.
The content
of soil organic matter varies, the mineralization is about 1-2% per year. Thus,
the contriburion of plant available nitrogen varies. It can be estimated by
measuring the nitrogen uptake in small plots sheltered from application of
nitrogen fertilizera, ”Zero plots”.
Great
efforts have been made to develop some laboratory method for determining the
mineralization, but so far there is no practical method available.
Some soils
have organic matter also in the subsoil, which somewhat contributes to the
nitrogen delivery. This will also be included by a Zero plot method.
Mineral
nitrogen.
Ammonium and
nitrate are also present in the soil. Excess fertilizer nitrogen or
mineralization in the autumn not absorbed by crops can result in considerable
amounts in the soil profile, contributing to available nitrogen for the next
crop, if it survives the winter. This might be important and there are methods
for measuring this factor and include it in the fertilizer recommendation
procedure (N-min, nitrogen profiles). It is important that the soil sampling
also includes the subsoil, to 60 or maybe 90 cm. This has been used in Swedish
agriculture, but it has been showed that for normal crops this component is of
less importance than variations in the mineralization. A zero plot method
includes also mineral nitrogen in the soil profile.
Nitrogen
forms.
In the agricultural
soils available nitrogen is converted to nitrate, often within a couple of
weeks. It is a microbial process, nitrification. With fertilizers nitrogen is
applied as ammonium, nitrate or urea. Nitrate is the final form in the soil in
any case, bur for ammonium and urea the availability is somewhat delayed. Urea
nitrogen might also be subject to losses by volatilization of ammonia if left
on the soil surface.
Manure and
other organic fertilizers also contain organic nitrogen. This must be
mineralized to be effective, which takes time. Alro for organic nitrogen the
final form in the soil will be nitrate. It should be mentioned, however, that
the plants can readily use ammonium nitrogen when the roots reach it. The
problem is the transport in the soil because ammonium is absorbed on soil
particles. Also small organic molecules may be absorbed by plants, but this is
of small practical importance in agriculture.
Biological
fixation of atmospheric nitrogen is more diverse than often believed. Legumes
are important for the agriculture, peas, beans, clover and luserne.
However, some ”freeliving” soil
bacteria also fix nitrogen, especially in the rhizosphere of plants. For rice,
sugar cane and grasses in the tropics this is of great importance. Symbiotic
nitrogen fixation provides nitrogen to the actual plant but with roots and
residues important contributions may be passed over to following crops. There
are some reports that in mixed stands with for instane legumes and grass som
nitrogen contribution is given to the grass.
Nitrogen to
the crop.
Proteins
and other substances containing nitrogen are important constituents in plants.
Cereal grains contain about 15-20 kg nitrogen per ton. A crop giving 6 tons
contains 120 kg nitrogen in the grain which is removed and in addition maybe 30
in the straw and 30-50 in the root system. In total 180-200 kg nitrogen is
engaged.
The soil
has a limited capacity to deliver nitrogen. The store may be large but the
delivering capacity is limiting. If the crop cannot obtain the 200 kg needed
there will be no 6 tons of grain, but less. In this way it can be said that
nitrogen availability governs the yield formation. But the nitrogen
availability can only constrain the yield, it cannot promote it above what is
allowed by other factors as water, climate, stand development, diseases etc.
The nitrogen fertilization should be adapted to the yield capacity of the stand
and the soil.
In the
present example the fertilizer application may be 120, the soil ontributes with
80. 120 is removed with the grain harvest. Straw and roots remain on the field
and pay back the soil contribution. In addition we have a contribution from the
atmosphere (10-20) , nitrate leaching (20-30) and also additional soil
mineralization after the ripening of the crop. There are also some nitrogen
losses by denitrification, which means that nitrogen gas and a small amount of
dinitrogen oxide (climate gas) is
released from the soil.
Some
important conclusions:
Nitrogen
availability may constrain the yield but it cannot lift it above what other
factors allow.
In well
functioning systems there can be almost balance between input in fertilizers
and removal by the harvest.
It is
necessary to keep in mind that surplus nitrogen is environmentally harmful.
A practical
situation.
The
following diagram describes the results from an experiment in Norway, as part
of a Scandinavian project (Lindén et al, 1992).
Increasing
nitrogen rates (horizontal axis) have affected the yield (vertical axis). The
experiment has been repeated 3 years, 1984 – 1986, on adjacent areas of the
same field . These 3 years and their average are shown in the diagram. The soil
and the crop (Spring Barley) is the same. Still, the yield results are very
different.
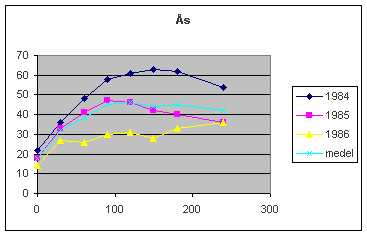
The optimum
for the average (price relations 10 kg grain pay for 1 kg N) is 90 kg nitrogen
and that fits well for 1985, But 1984 the optimum is 120 and 1986 only 30 kg
nitrogen. The reason is not differences in the soil delivery. There are only
small differences in the zero treatments, and should we consider those more
nitrogen should be needed in 1986, quite the wrong direction. Other
unfavourable circunstances depressed the barley in 1986 and then more nitrogen
fertilizer does not help at all.
This is a
very evident example of the problems encountered in practice. The weather,
diseases (1986 is third year barley), weeds, problematic establishment etc
affect the crop. Averages from experiments give a general guidance but we saw
in the example above that it did not work well in 2 years of 3. But now better
methods are available for adapting the nitrogen rate the individual year. By
means of sensor technology the application rate is adjusted according to the
state of the crop and as it varies in the field. This involves the following
steps:
- Study the crop history of the
individual field: yields, protein contents, observations, fertilization.
Zero plots or -observations are valuable.
- With this background, estimate
the yield goal and corresponding probable nitrogen demand.
- Plan a two-step strategy. Apply
50-70% of estimated demand at ”normal” time, make a complement later,
preferably with sensor guidance. But even without sensor this two-step
strategy is important. Stand observations (preferably plant or tiller
counting) and the seasonal climate may give background for adjusting the
previously planned fertilizer rate.
In this way
unnecessary overdoses can be considerably reduced the problematic years and the
high yield potentials fulfilled the good years. There are economic gains,
although these should be weighed against the costs and trouble invoked by the
two-step method.
The
environmental aspect is important as will be expanded below. In the practical
example described above in 1986 about 100 kg residual nitrate was found in the
soil. Most of that will be lost to the environment in different ways and cause
both local and global problems.
Nitrogen in the environment.
Nitrogen
leaching gives eutrophication in streams, lakes and seas (together with
phosphorus).
Ammonia
losses distribute nitrogen in nature and increases eutrophication in general.
Nitrogen
turnover in soils and waters produces some dinitrogen oxide, which is a
powerful climate gas and also a threat to the ozone layer.
In general,
the human society should aim at reduction of the nitrogen flows. This is a hard
task as we have an expanding population in itself needing more and more
nitrogen. It is really an urgent task to use nitrogen efficiently in all
respects. The agriculture has an important role here.
Preliminary
ecological estimates say that the global nitrogen flows are too large already
today. The global ecology is negatively affected.
Nitrogen
has many ways. An example. An overdose of nitrogen increases residual nitrate
which is leached to rivers and the sea. Plants and algae are fertilized and
increase. Eventually the nitrogen is released, and some of it is in the form of
ammonia och dinitrogen oxide affecting the environment. The nitrogen surplus
also gave higher nitrogen concentration in the soil giving increased formation
of dinitrogen oxide there, and also higher nitrogen content in straw and roots,
which also increase
these losses. Further, the harvested grain
will have unnecessarily high protein content, which might increase losses in
the utilization chain. Still further, the production of the unnecessary
fertilizer led to unnecessary emissions of climate gases and unnecessary energy
use. This is an example of a cascade effect. Introduction at one point leads to
many consequences downstream in the chain .
We need to
straighten up nitrogen use where we can.
Marginal
effects of nitrogen.
For
especially nitrogen the marginal economy is often stressed. You shoull apply up
to a level where the cost of an additional kg N is compensated by the value of
the yield increase. Especially at high product prices this is almost a
dangerous principle. With the balance principle discussed above we avoid this.
But the marginal philosophy is one of the ground pillars in the economy of
today and it is also extensively used for calculation of nitrogen optima. So –
let us take it one step further and look at marginal ecology..
We use the
practical example from above: the experiment at Ås, Norway. We use the results
from 1984, the best year.
GHG =
GreenHouse Gases, carbon dioxide equivalents. Nitrogen fertizer
manufacture+turnover in soil
Prices: SEK
12 per kg N and 1.90 per kg grain. Relevant for Spring 2011. Net is grain value
minus fertilizer cost.
|
N rate |
Yield |
SEK/ha net |
GHG kg |
Leach. Kg N |
Energy GJ/ha netto |
|
Aver. per kg grain |
Stepwise per kg |
|||
|
GHG |
Leaching |
GHG |
Leaching |
||||||||
|
0 |
2200 |
4180 |
582 |
23 |
21 |
|
0,26 |
0,010 |
|
|
|
|
30 |
3600 |
6480 |
906 |
23,5 |
37 |
|
0,25 |
0,007 |
0,23 |
0,04 |
|
|
60 |
4800 |
8400 |
1218 |
24 |
50 |
|
0,25 |
0,005 |
0,26 |
0,04 |
|
|
90 |
5800 |
9940 |
1518 |
25,6 |
61 |
|
0,26 |
0,004 |
0,3 |
0,16 |
|
|
120 |
6100 |
10150 |
1776 |
30 |
63 |
|
0,29 |
0,005 |
0,09 |
1,47 |
|
|
150 |
6300 |
10170 |
2028 |
37 |
65 |
|
0,32 |
0,006 |
1,26 |
3,5 |
|
|
180 |
6200 |
9620 |
2262 |
52 |
63 |
|
0,36 |
0,009 |
|
|
|
|
240 |
5400 |
7380 |
2694 |
83 |
51 |
|
0,5 |
0,015 |
|
|
|
With the
prices given 150 N is the most economical treatment. The Balance principle would have given about 110 as recommendation.
What
happens when you go from 120 to 150?
The
economic gain is SEK 20 (less than the value of 2 kg N).
GHG
increase from 1776 to 2028, 252 kg. That is more than the normal emissons from
the tractor per hectare. You get 200 kg in increased production which means
1,26 GHG per kg grain. This is more than would be expected from plowing up old
grassland. The actual Swedish carbon dioxide tax is SEK 3000 per cubic metet
oil, which means about SEK 1 per kg GHG. If we use that figure we have a cost
for climate gas emission of about 250 for this step. The leaching is estimated
to inrease by 7 kg N. In other situations the society is prepared to pay SEK
50-150 per kg N leaching reduced. If we use 50 we have a leaching cost of 350.
The
fundamental economic maximization led to a gain for the farmer by SEK 20 and
emission costs for the society of more than 500. If we scrutinize the table we
see that around 100-120 kg N gives a good combination of economy and ecology.
There is a very small theoretical loss
of income which should be discussed with the farmer.
We should
not over-emphasize marginal economics for fertilizer recommendations, There are
traps in the system, especially when broad averages and response functions are
used. We should look more to the actual demands and use either a balance
principle with adjustments ot, preferably, a stepwise practice with sensor
guidance. Methods and technology are available.
More details from experimental data.
Nitrogen and
soil fertility.
In Swedish
agriculture nitrogen fertilization is a positive factor for soil fertility. It
gives high yields and also increased production of crop residues and is
positive for soil organic matter. All longterm experiments give evidence in
this direction.
But you can
of course speculate: how would agriculture work without nitrogen fertilizers?
There would have been more leys, more direct combination of animal and crop
production. Lower intensity, lower yields, much lower production. There would
probably be some advantage as concerns landscape and diversity. But it is not
without reasons we have had the development we have seen. The reasons are at
least two: rural standard of living and feeding the urban society at reasonable
cost.
It is possible
that we had a low mark concerning soil fertility development during the 1960s
and 1970s. Straw burning was common, manure was not used optimally, the yields
were mediocre. The agriculture of today
with high yields and proper residue management has changed that.
There is
another step to take. With optimal use of cover crops and reduced tillage we
can improve soil fertility and create resources to better take care of
biodiversity and landscape issues.
There are a
few reports internationally that nitrogen fertilizer reduces organic matter and
soil fertility. Of course this may happen in cases where the soil microbial
community is nitrogen starved, or when cropping with legume cover crops is used
as comparison. In northern European conditions nitrogen is a positive factor,
but this does not mean that considerations concerning rotation and soil organic
matter maintenance can be forgotten.
Nitrogen as
a resource.
Nitrogen as
such is no resource issue. The reserve, nitrogen in the atmosphere, is
inexhaustible. However, it is an issue of energy and environment.If it costs
more energy and emissions to recycle nitrogen than to produce new fertilizer
recycling is unfavourable. If cropping with biological nitrogen fixation in the
end costs more energy and emissions than more intensive fertilized systems it
is of no general advantage. Such comparisons must be made considering the whole
system, and there is no general answer. When comparing nitrogen fertilizer with
legume nitrogen you get one result in Sweden and probably another for
smallholders in inland Africa.
Often in
the general discussion a reduction of nitrogen fertilizers is seen as a goal in
itself. This is logical only if there is a general over-use of fertilizers. If
that is not the case there is the question about the consequences of the impact
of alternative nitrogen sources or of decreased production. The crops, the food
production, need nitrogen, and the task
is to manage this as well as possible considering all aspects.
If we
compare organic agriculture with properly managed normal agriculture no
advantages have been shown for the organic. But there are some key words:
”properly managed”. That is not always the case. Let us work on it.
One
important issue on comparisons. The nitrogen industry has improved considerably
the last few decades. The energy consumption and the emissions have been
greatly reduced. Many ”old truths” are not true anymore.
On energy:
nitrogen fertilizer is in fact an energy source, or an energy amplifier.
Properly used one kg nitrogen in fertilizer (energy cost 35 MJ) gives at least
30 kg dry biomass (practical energy content about 12 MJ per kg). This means an
amplification of about a factor 10, marginally. If we upgrade this bioenergy to
refined biogas there is still an amplification factor of 4-5.
Phosphorus.
Phosphorus
in the soil.
A normal
Swedish soil contains 1 -2 tons of phosphorus in the top soil layer. In Sweden
the P-AL method is used for estimation of ”available phosphorus” and P-HCl for
”reserves”. These fractions are in certain equilibrium. In addition there is still less soluble phosphorus in minerals and in soil organic
mattter.
Long term
experiments give evidence about the soil P dynamics. Here is an example from the soil fertility experiment Orup in
South Sweden.
The
treatments are as follows:
A. Without
P
B.
Replacement of exports according to analyses.
C. R + 15
kg P/hectare and year.
D. R + 30
kg P/hectare and year
A
comprehensive soil analysis was made after 27 years. We know that D has
received 810 kg P more than B and C 405 more than B. The export from A is about
400 . What do we find?
The
following figures are expressed as kg/hectare in the topsoil. Order: Organic P,
Reserves, Available and Sum.
A: 989,820,50, sum 1850
B:
1040,860,100, sum 2000
C: 1040,
1060, 200, sum 2300
D: 1060,
1260, 320, sum 2640.
In D, 810
had been added compared to B, 220 is found in Available, 400 in reserves and 20
in Organic, sum 640. Some would have been brought to the subsoil by rroots.
There are of course uncertainties in soil sampling, soil density estimation and
analysis, but the main conclusions are crystal clear.
We see an
interaction between Reserves and Available. The reserves are truly active, take
care of surpluses and deliver when needed.
Leaching
and other losses? The mean phosphorus loss from Swedish agricultural soils is
0.3 kg per hectare and year. In 27 years that would mean less than 10 kg, to be
compared with the figures above. These losses are unimportant for the soil and
agriculture but have a large influence on water bodies.
This
picture seems to be normal for most soils. We can use the reserves as a buffer,
consider the phosphorus fertililization over a rotation period and apply were
most efficient.
Phosphorus
to the crop.
A good crop
takes up about 25 kg P, and as we discussed above there is more than a ton in
the topsoil only. In addition there are considerable amounts of P in most
subsoils. Why is it necessary to add P in fertilizers? But experiments, also
the Orup experiment we discussed above, show high yield increases for
fertilizer P. It is not the total amount that matters, it is the availability
which in Sweden is estimated by the AL method.
There are other methods elsewhere.
As a rule,
the plant roots must seek out the phosphorus. The concentration in soil water
is very low, and the phosphorus in the water the roots take up is completely
insufficient. But when the root tip is boring its way in the soil it sends out
root hairs which make close contact with soil minerals and absorb P. A kind of tunnel of P depleted soil is
established around the root. So, it is understandable that the root development
is important. Mycorrhiza can add considerably to the contact plant – soil.
Crops
differ in ability to utilize soil phosphorus. Grasses, clover, rye and winter
wheat are are fairly efficient. Beets, potatoes and maize are less efficient
and have greater demands and higher fertilizer requirements. Spring cereals and
oilseeds are in between. New varieties and cultivation techniques influence
recommendations and continuous updating is necessary.
Phosphorus
fertilization should be planned with long term consideration of the change in
soil P pools. For example, winter wheat takes up about 25 kg P but can use soil
P if the values are not too low. The direct response to additions in
fertilizers is low. But the barley or rape which may follow respond better and
it is more favourable to concentrate the phosphorus fertilization to these
crops. An optimizaton for the whole crop rotation is advantageous.
Phosphorus and environment.
The
phosphorus outflow to the Baltic Sea is seen as one of the main environmental
problems caused by agriculture. Still, the specific loss of P from agricultural
soils is low, about 0.3 kg P per hectare and year in average. But P is strongly
affecting water bodies. The agriculture should do what is possible to further
reduce the outflow of P.
Part of the
outflow is caused by erosion, soil material carried away by flowing water.
Measures as keeping a vegetation cover in the autumn and winter, leaving harvest residues on the soil surface
and adapting the soil management are important.
Plant
material may release P, especially after freezing. Here we see a conflict.
Plant residues on the soil surface reduce erosion but may increase P losses
from the residues themselves.
Some P is
dissolved in water. The concentration is partly dependent of the soil P status.
Above P-AL 10 (mg P/100 g soil) there
is a marked increase of the P concentration in outflowing water. For
environmental reasons this level should not be exceeded. Is this a constraint
for agriculture? The answer is no. For all normal crops we have full production
at P levels below P-AL 10. However, farms whith animal production have manure
to take care of and that complicates the issue. In Sweden there i a limit of 22
kg P per hectare in animal manure as average over the farm. That gives a
certain balance but not always possibilities to adjust an unnecessary high
phosphorus status.
If the
phosphorus status is suitable for the cropping system it has to be maintained
by replacing the export. It seems that replacement is suffient on most soils.
However, certain soils may continue more than replacement to keep the status as
measured by P-AL and monitoring by soil analysis is necessary. Some decades
ago, during 1950-80, we had phase of soil fertility build up. Recommendations
considerably higher than replacement were used at that time.
It is
important that the P fertilization is done efficiently. Naturally, it is
positive to get as high yield increase as possible for the maintenance
fertilization given. Placemant is an efficent method especially if NPK
fertilizers are used.
Phosphorus
and sustainability.
Phosphorus
is essential for life and nothing can replace it. The discovery of the secret
of plant nutrition gave us P fertilizers and the possibility to increase the
crop production and secure the food supply. It is hard to see that our society
can continue development without having contros of the P supply.
Phosphorus
is a fairly common element, but it is tied up in minerals mostly in low
concentrations. The richer deposits we know of today do not last for ever. If
the most recent estimate of reserves is divided by the present consumption we
arrive at about 400 years. In addition there are known resources which are more
expensive to use but give the figure 2000 years. The estimates of both reserves
and resources have been considerably increased recently. Previous alarm reports
saying the phosphorus sources are depleted in about 100 years are not valid any
more. If the phosphorus costs rise more sources will be economical to use.
The
phosphate deposits contain many elements, also some we do not want to have in
fertilizers. Some purification process will be increasingly necessary. Pilot
scale data indicate that this is not a critical issue for the P supply.
Nevertheless,
it seems important to economize with our phosphorus reserves. To continue the
single direction flow of P from agricultural soils to waste deposits and waters
is not sustainable. Only a fraction is recycled today.
Another
factor is the continuous enrichment of the biosphere. Phosphorus is a very
active element and fetching it from locked geological reserves to the biosphere
has consequences. Recycling would reduce this flow.
So – in
spite of the upgraded P reserves improved recycling should be developed and
encouraged. There are some alterntives and more in development.
Potassium.
Potassium in the soil.
Potassium
is an exchangeable ion and is temporararily adsorbed on the surface of mineral
particlis. It is also a constituent of soil minerals, and especially clay soils
contain large amounts of potassium which can be successively released. When the
plant takes up potassium, soil solution and exchangeable K are used firsthand.
This causes background reserves to be mobilized. Clay soils can deliver K
during long time, depending on clay minerals.
Available K
is in Sweden estimated by means of K-AL, which roughly corresponds to
exchangeable K, the reserves by K-HCl.
For
potassium the reserves do not function well as a flexible buffer. Soils low in
K usurally are poor in clay and potassium surplus is prone to leaching or
luxury uptake by the crop.
Potassium
to the crop.
The plants
need substantial amounts of potassium, 100-300 kg K per hectare. Potassium is
present mainly in the vegetative parts of the crop. If only the grains are
harvested only a few tens of kilos are removed, with straw harvest considerably
more. High yields of leys remove large amounts of K and the balance needs
consideration. 10 tons of crop dry matter contains at least 200 kg potassium.
Like for
phosphorus the uptake of potassium from the vicinity of the roots is important.
Even if there are large reserves and the removal is small the acute demands of
the crop need to be satisfied for a good yield. The fertilizer recommendations
are based on field experiments and consider this aspect.
Potassium
interacts with other nutrients, especially magnesium. Potassium fertilizer may
increase the risk for magnesium deficiency. This interaction is considered in
many fertilizer recommendations, the K/Mg ratio.
Recent
experimental results in Sweden show unexpected yield increases for placement of
NPK compared to placement of NP also in soils with high K status. The reason is
not clear. Speculations:
It may be
an advantage if all nutrients are available in the same soil volume, especially
for rapidly growing crops as spring barley.
Another
possibility is a direct or indirect chloride effect. We talk about potassium
addition, but in fact nearly the same amount chloride is added if normal
fertilizers are used. It can influence both the plant and the transport in the
soil.
Our
knowledge is incomplete. The work must go on.
Potassium
and the environment.
No
environmental problems caused by potassium in agriculture are reported.
Potassium
and sustainability.
The raw
material for potassium fertilizers are geological deposits of salts. The
reserves are large.
Magnesium.
Magnesium
in the soil.
Magnesium
is an exchangeable ion, just like potassium and calcium. It can be stored in
the soil, better the higher the clay content. Soil analysis: The Swedish method
is Mg- AL, which roughly corresponds to the exchangeable K.
Magnesium
containing lime is a suitable magnesium source for soils needing lime. Some
crops may benefit from extra magnesium in fertilizers.
Magnesium
in the crop.
Magnesium
is a central building block for chlorophyll.
Sulphur.
Sulphur in
the soil.
Sulphur is
present in organic substances and as sulphate. Microbes transform organic
sulphur to plant available sulphate. Sulphate is weakly absorbed in the soil
and is lost by leaching, although more slowly than nitrate. Sulphate cannot be
stored in the soil and must be considered on annual basis. Some decades ago air
pollution gave so much sulphur to air and precipitation that sulphur deficiency
was rare. This pollution is now so much reduced that it is important to
consider the sulphur situation when planning the fertilizer use.
Sulphur in
the crop.
Sulphur is
a constituent in essential proteins. The plant content is in the same size of
order as for phosphorus.
Sulphur and
environment.
Sulphur
from normal agricultural soils give no environmental problems. It is not
acid-forming. However, acid-forming sulphur in air and precipitation causes
acidification of soils and waters. The great threat is now very much
reduced, but some sensitive areas are
still affected.
Micronutrients.
For North
European conditions mainly manganese, boron and copper are of practical
importance. For boron and copper soil analyses are available
The
availability of manganese and boron is reduced at higher soil pH values.
General issues of plant nutrition.
Focus on the actual crop or the soil for
fertilizer planning.
The actual
crop: nitrogen, sulphur, manganese.
The crop in
combination with soil status: phosphorus, potassium, boron.
Mainly the
soil: magnesium, copper
Foliar application.
Plants can
absorb small amounts of nutrients via the foliage. It works best for
micronutients as manganese and boron. Smaller doses of nitrogen can also be
used, in which case urea works best.
Timing and application.
Mostly it
is most efficient to apply nutrients in close harmony with the crop demand,
that is in Spring or during the growing season. Autumn application av phosphorus
and potassium has been common, but there is a drawback of increased leaching
losses of P and on light soil losses and reduced effect of potassium. NPK in
Spring is more efficient, especially as placement or side-dress.
Long term consideration.
This is
mentioned earlier, but needs to be stressed again. Agriculture should not be
only a shorttime business. Agricultural advice and research should focus on
helping agriculture with more longterm considerations. It could be about crop
rotations, soil organic matter, soil management or the whole organization of
the farm production. It is important, in fact necessary, to put prices on as
much as possible, for instance rotation
and soil organic matter effects and include this in the economic
planning.
There is at
least a start in the advisory programme Focus on nutrients.
Crop rotations, soil organic matter and soil
life.
Data from
soil fertility experiments.
We have in
Sweden 8 so called Soil Fertility Experiment, now about 50 years old. There are
2 rotations, one with ley and manure, and one with only crop production. There
are also 4 levels of nitrogen and 4 of phosphorus+potassium, all in a complete
factorial arrangement. Let us look at time series for crops in common for the
two rotations, cereals and sugar beets. We see that the yields in the rotation
without ley is lagging behind in most experiments. The difference is now
between 4 and 9 percent in different experiments, which means an income
difference of more than SEK 1000 per hectare, and the trend is increasing.
However, this difference is not found in experiments where the soil organic
carbon content is more than 2%.
How can we
interprete this? It is no nitrogen effect, which we can deduce from the
different nitrogen treatments as well as from protein contents in the crops. Is
it pure rotation effects of the ley or a special effect of manure? If so – why
not also at the sites with higher soil carbon? There is a small difference in
organic carbon between the systems, a couple of tenths of percent after 50
years of different systems. This shows that there is a difference in soil
carbon management and consequently in soil life.
Soil life.
A plausible
interpretation of the results:
Soil life
is more important than generally appreciated. Soil life gives soil structure
stability and robustness. And if soil life is maintained automatically the soil
carbon management is improved. Visible changes in soil carbon content takes a
long time to establish, but only by working towards that goal there is a reward.
The practical road-map:
recycling
of harvest residues
leys
manure etc
cover crops
or autumn crops keeping up production of organic materials also in the autumn
reduced
soil management.
Approximate
”critical level” for soil organic carbon: 2% C.
All Swedish
soil fertility experiments which are below 2% C give yield increases for improved soil carbon management as
mentioned above.
An
international scientific summary report about ”critical soil level” arrived at the figure 2%.
The
interpretation should not be that soils below 2% C are inferior to others. The
fact is that in Sweden our most productive soils are low in carbon. But they
perform still better with improved carbon management.
High-yielding soils are most dependent on good
carbon management and soil life.
This is not
what would be expected, firsthand. However, it could be seen like this: To get
810 tons of grain everything must be on top the whole vegetation period, from
establishment to grain filling. Neither water nor nutrient should ever be critical.
Carbon management and soil life helps.
If 5v tons
is the normal yield there are more serious limitations, perhaps the soil
profile, which is not easily improved.
Mechanisms för soil organic matter and soil
fertility.
Textbooks
often say that organic matter helps with water and nutrient management. This is
true to some extent but it is not enough. At least for clay soils the effects
on soil structure is most important. And that is an effect caused not only by
soil organic matter but of soil life. It gives hyphae stabilizing soil
aggregates, it gives polysacharides helping with the same thing. In reduces
aggregate distruction, increases permeability and aeration.
Cover crops and soil organic carbon.
The Swedish
catch crop programme is about catching nitrogen. It should be caught in organic
material, in the catch crop growing. When the catch crop is plowed under the
soil gets an addition of organic material. A secondary effect is improved soil
carbon management. What does that mean? Evidently, it depends on the amount of
catch crop material. But it should be noted that in addition to the vegetation
we see there is an extra root system which means a lot. Further, a catch crop
often means thet the soil management is delayed, which reduces the mineralization
of soil organic carbon.
Internationally,
there is a important work on cover crops and soil fertility: Denmark, Germany,
France, USA. In Norway experiments show important soil carbon effects which
gives promise also for Middle Sweden.
One could
wish for more data from Swedish experiments. Unfortunately, there are no
reliable soil organic carbon data from Swedish catch crop experiments, and many
years are needed for such work. But there is the general base for soil organic
carbon development, supported by the international work mentioned above.
Soil
organic matter, soil carbon management and the environment.
Soil
organic matter improvement is environmentally advantageous, as long as it is
not excessive. It increases the stability of the soil, reduces soil erosion and
in consequence phosphorus losses.
Excessive
soil organic matter improvement is almost a theoretical issue for the crop
producing agriculture. But if the soil is used for deposision of organic refuse
the nitrogen losses might increase. Animal intensive agriculture may approach
this situation. But improved carbon management in crop producing agriculture is
as a whole environmentally positive.
There are
details to discuss, as early incorporation of nitrogen rich organic material,
early killing of catch crops etc. Carbon can be managed more or less
environmentally friendly.
Agriculture
and energy.
This issue
is a pedagogical challenge. The food chain as a whole is not energy efficient.
Roughly one tenth of the input energy ends up in the food we eat. One main goal
of the food production is to give energy to teh population, and against this
background the effeciency is regrettable low.
Let us see
what happens in different steps of the food chain. We assume an input of 100 MJ
as fuel and fertilizers.
The crop
production gives about 6 times the input, so we get 600 MJ in plant products.
The animal
production consumes energy. We get meat and milk, but only 150 MJ remains
Then we
have food industry, logistics, trade and cooking. We get prepared food but only
20 MJ remains.
20 MJ can
be put on the table. But there are remains and losses, and only half of this is
actually consumed.
Which means
10% of the input.
But the odd
thing is that ”the energy demanding fertilizers” often are blamed for this
inefficiency.
Let us
instead look at prospects.
With one kg
of nitrogen in fertilizers, which has cost about 40 MJ to manufacture, we
produce 30-50 kg extra plant matter. Let us assume 30. Total energy content
about 500 MJ. We assume that it is used for producing refined biogas. With
available technology we get 250 MJ of biogas. We take 40 MJ to compensate for
the fertilizer and 210 remain for the society.
Nitrogen
fertilizer, properly used, helps catching solar energy and is a kind of energy
amplifier. By means of biogas or other bioenergy technology agricultural lands
can be an important energy producer. And – that can be achieved without
compromising the ordinary agricultural production. Three resources are
available in addition to the system of today;
- Cover crops giving an extra
production in the autumn. In south Sweden 3-4 tons dry matter can be
produced with proper management.
- Cover crops are incorporated in
the soil and helps up the soil carbon balance to the extent that harvest
residues can be used for bioenergy.
- Break crops for bioenergy in
the crop rotation. They cost a year of ordinary agricultural production,
but give important compensation by improving yields of the other crops.
It might be
added that energy production (ley harvest) can be possible on marginal lands
where open landscape is a part of the production. This could be a complement to
grazing animals.
1 000 000 ha * 2000 kg * 2 kwh = 4000 000 000 kwh =4 Twh
Biodiversity.
The very
idea of agriculture is to replace the natural diversity of plants with i crop
which can be better utilized by man. Main task: to fight biodiversity, at least
on the cultivated fields.
However,
biodiversity is of value for the society and is one of the Swedish
environmental goals.
It has also
some positive components for agriculture: pollination, more robust biological
systems